Tumor Growth Pattern is Significantly Associated with Metastasis in Patients Diagnosed with Colon Carcinoma – A Computer Image Analysis Study
Abrar Ahmad and Victoria Hahn-Stromberg
DOI10.21767/2572-309X.100011
Abrar Ahmad1 and Victoria Hahn-Stromberg1,2*
1Department of Clinical Research, Faculty of Medicine and Health, Orebro University, 70185 Orebro, Sweden
2Department of Medical Cell Biology, Uppsala University, 75105 Uppsala, Sweden
- Corresponding Author:
- Victoria Hahn-Stromberg
Department of Medical Cell Biology,Uppsala University, 75105 Uppsala, Sweden
E-mail: victoriastromberg@hotmail.Com
Received date: April 08, 2016; Accepted date: May 13, 2016; Published date: May 20, 2016
Citation: Ahmad A, Hahn-Stromberg V. Tumor Growth Pattern is Significantly Associated with Metastasis in Patients Diagnosed with Colon Carcinoma – A Computer Image Analysis Study. J. Adenocarcinoma. 2016, 1:2. doi: 10.21767/2572-309X.100011
Abstract
Background and aim: Growth pattern of the tumor has been studied for its association with survival in colorectal cancer. Among different growth pattern evaluating techniques, very little is known about prognostic significance of complexity index. The aim of this study was to develop a prognostic model, which could be used to predict prognosis in the patients diagnosed with colon carcinoma. Materials and methods: Formalin fixed paraffin embedded tissues samples from 316 patients diagnosed with colon carcinoma were used in this study. The slides were stained for cytokeratin-8 and images of the invasive front of the tumor were captured using a camera mounted onto a microscope. Images were thresholded in order to get the tumor area black and the surrounding tissue white. The tumor outline was also highlighted as a single pixel line to calculate the fractal dimension and number of tumor cells and tumor cell clusters. These two features were then used to calculate the complexity index by performing a tree diagram analysis. Complexity index was correlated with 5-years survival and other clinicopathological data (age, gender, tumor penetration, lymph node metastasis, duke’s stages, anatomical localization and differentiation of the tumor) of the patients. Results: Clinicopathological parameters like tumor metastasis, localization, gender and differentiation were significantly associated with tumor complexity index with p=0.000, p=0.002, p=0.024 and p=0.000 respectively. A positive trend was also observed between complexity index of tumor and age variable (p=0.051). Tumor wall penetration, lymph node metastasis and Duke’s stages were not significantly associated with tumor growth pattern; complexity index with p=0.997, p=0.857, p=0.783 and p=0.647 respectively. Conclusion: We conclude that complexity index is associated with systemic metastasis and tumor differentiation in patients diagnosed with colon carcinoma. Complexity index is a reliable objective and quantitative technique used to analyse tumor characteristics and can be used as a prognostic tool in the individual treatment of colon carcinoma
Keywords
Colon carcinoma; Prognosis; Immunohistochemistry; Biomarker; Tumor growth pattern
Introduction
Colorectal cancer (CRC) is one of the most important public health problems and one of the leading causes of cancer mortality in industrialized countries. There are about one million new cases of CRC worldwide with half a million deaths per year [1]. In cancer related deaths, colorectal cancer (CRC) is at 4th position in men and 5th among women [2]. CRC is considered 3rd most frequent type of cancer worldwide with 90% survival rate at stage 1 and only 10% in metastatic cancer [3]. About 25% cases are metastasized to the liver at the time of diagnosis that can be treated surgically but within 2 years, two thirds of them are relapsed [4]. Nevertheless, curative surgery is the most important aspect of treatment. In selected patients whom liver can be amenable to surgical resection, 5 years survival rates are 35-40% but prognosis is very poor for those whose liver metastasis is unresectable [5].
Over the last two decades, CRC patients have improved 5-years overall survival. Some patients at advance CRC stages got benefits of survival by resection of liver metastasis and improvement in surgical methods. Although overall survival in advance CRC patients is lower than those who are at early stage but it is encouraging to find the outcome prediction of CRC patients to device an appropriate therapeutic strategy [6].
Consequently, many researchers have focused on identifying biomarkers that could be used to predict the biological behaviour as well as response to therapeutic implications in CRC. There are several histological variables, that are identified as predicting factors in disease development. They include depth of tumor invasion, degree of tumor differentiation, presence of metastasis and indication of perineural or perivascular tumor invasion [7]. In CRC patients, tumor growth pattern is considered as an indirect factor that predicts the tumor recurrence [8]. Mounting evidence suggest that growth pattern of the tumor has information about the metastatic ability of tumor; more irregular shape of the tumor invasive front will be responsible for high metastatic potential and vice versa [9,10]. The disintegration of tumor cells in the invasive front showing an infiltrative type of growth also called tumor budding, where high budding has been significantly associated with lymphovascular invasion and low survival in CRC patients [11,12]. So it is important to uncover the biological events, which are occurring during CRC metastasis and formulate some strategy to intervene in this process.
In 1986, scientists described two types of tumor growth patterns, expansive growth with a smooth tumor-stromal front and an infiltrative growth in which the invasive front of the tumor splits into clusters [13]. In 1987, Jass et al. identified the invasive growth pattern as an important prognostic marker in colorectal cancer and found that the infiltrative type has worse prognosis compared to tumors with an expansive growth pattern [10].
Jass classification was revisited to see the intra-observer and inter-observer reproducibility in 1994, which shows slight to fair agreement with kappa values of 0.37 and 0.40 respectively [14]. These unsatisfactory results between single and multiple observers for classification of growth pattern in CRC evoked the importance of objective methods to be used for analysing the tumor growth pattern that could be gauged by considering some dependable variables of the tumor.
Naturally, all biological objects including tumors show fractal geometry and grow according to fractal rules and so it is a more valid method to measure complex irregular objects than other integer dimensional geometrics such as Euclidean geometry in which growth is described in one, two or three dimensions [15]. In different areas of biology like molecular biology, vascular and tumor pathology, fractal geometry is efficiently used [16]. In pathology, fractal dimensions have enabled to differentiate between tubular, tubuvillous and villous adenomas of the colon. Similarly it is used to distinguish severe dysplasia from benign conditions in epithelial connective tissue interface in the floor of the mouth [17,18].
In a previous study by Franzen et al. a computer-based technique called “complexity index” was developed which objectively measures the complexity of a tumor by measuring fractal dimensions and number of tumor cells/clusters [19]. These two variables were selected to quantitatively measure tumor growth pattern as its simple to analyse without any notable loss of precision. Previously, complexity index has been studied for its correlation with different cell membrane proteins and clinicopathological parameters of the patients to discover any significance association [20-22].
To our knowledge, no study has been made which analyses the tumor growth pattern by complexity index and addresses the association between complexity index and prognostic factors of patients diagnosed with colon carcinoma. The purpose of this study was to investigate the reproducibility and prognostic significance of complexity index correlated to the tumor growth pattern and clinicopathological parameters like age, gender, TNM (tumor penetration, lymph node metastasis, systemic metastasis) duke’s stages, anatomical localization and differentiation of the tumor of patients diagnosed with colon carcinoma.
Materials and Methods
Patients and clinicopathological data
A total of 316 patients diagnosed with colon carcinoma that underwent surgery from 2002-2009 at Orebro University Hospital, Orebro, Sweden were selected for this study. The patients who were having conditions predispose to CRC (e.g., inflammatory bowel disease, preoperative chemo-radiotherapy) were excluded. Patients who died as a complication from surgery were not included in this analysis as their death was not related to tumor biology and can introduced a confounding influence on survival results. Cases were anonymized and assigned serial numbers along with year of diagnosis. Clinicopathological data of the selected patients including age, gender, tumor penetration, lymph node metastasis, systemic metastasis, anatomical localization of the tumor, tumor differentiation and duke’s stages was extracted. The survival data of the patients was also obtained from the patient register at Orebro University hospital, Orebro, Sweden. Age variable was divided into two groups, below 70 years or above 70 years. This study was approved by the Ethical review board, EPN, Uppsala, Sweden.
Sample preparation
Formalin fixed paraffin embedded blocks from patients diagnosed with colon carcinoma were used for this study. For each patient, a tumor block was selected. These blocks were incubated on ice for at least 15 minutes prior to sectioning. A microtome (LEICA RM 2155) was used to obtain 4 μm thick sections of the block. The sections were mounted on immunohistochemistry (IHC) glass slides (Superfrost® plus-Thermo scientific) which were then dipped in hot water at 50°C in water bath device (Kunz Instrument, Histolab). Samples are collected and heated at 62.3°C for 1 hour in oven (Nuve, EN400, Lab Klimat AB) and left at room temperature to cool.
Immunohistochemistry staining
Slides having sections were pre-treated with EDTA buffer saline solution (En Vision™ FLEX Target retrieved solution, Dako, high PH 50x) at 97°C for 1 hour. Slides were subsequently washed and stored in buffer (En vision™ FLEX wash buffer 20x, PH 7.75) until staining. Staining was performed by using Dakos Techmate and DAB Envision following the manufacturer’s protocol (Dakocytomation, Denmark). The primary antibody, monoclonal anti-Cytokeratin (clones AE1/AE3, dilution 1:50, Dako, Denmark) was applied to all slides for 30 minutes at room temperature. Mounting was performed after immersing slides into ascending ethanol concentrations and xylene.
Computer image analysis
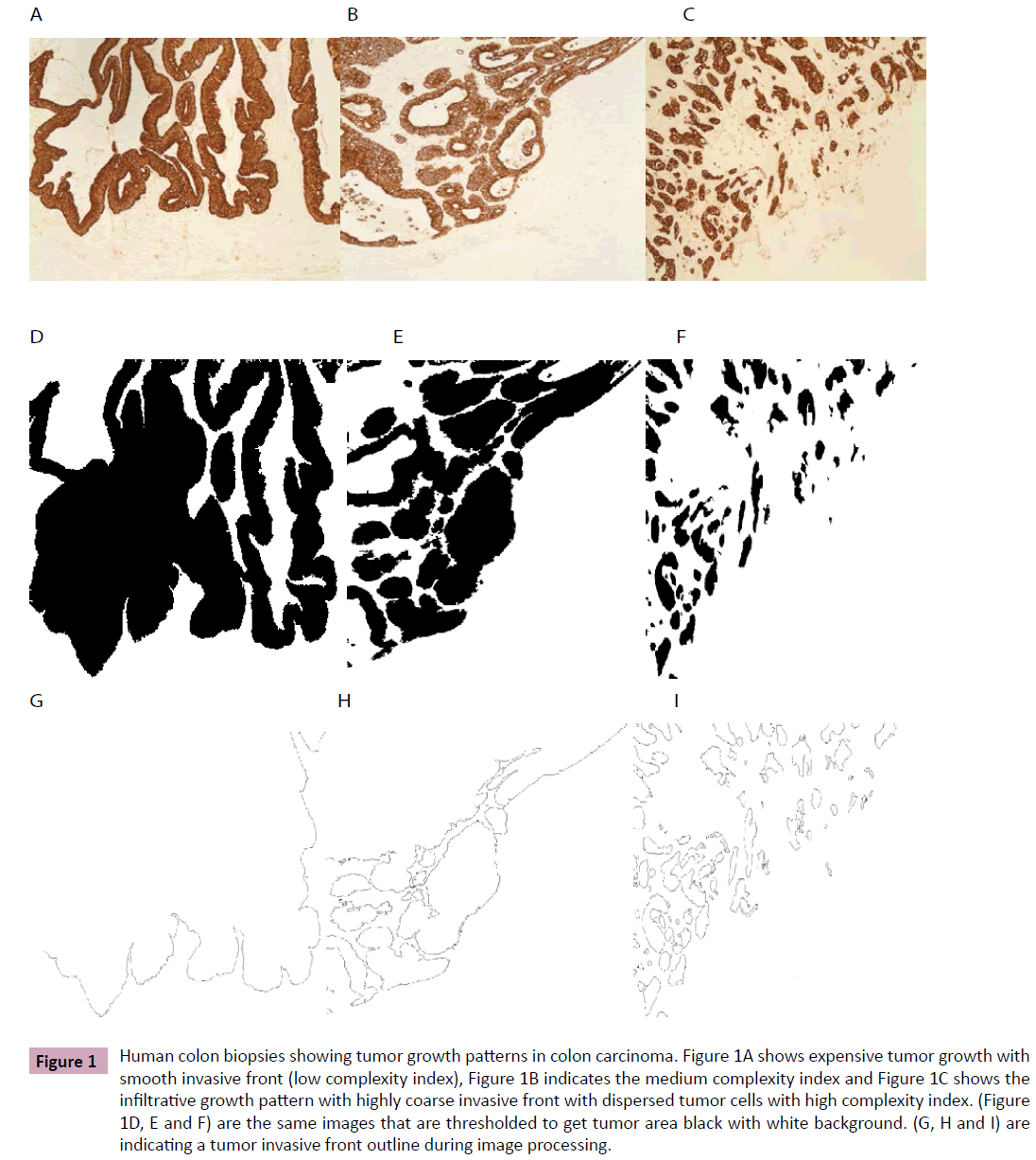
Images were captured from the tumor-stromal area of the tumor and processed by the same method as described by Franzen et al. [19]. Briefly, images were captured by a camera mounted over the Leica DMRXE microscope (Leica Microsystems Wetzlar GmbH, Germany) at 10X objective. These images were threshold to get a) tumor area black to measure fractal dimensions and b) tumour cells border marked black to count the number of cells/ clusters. These two characteristics of the tumor were used in tree analysis to get a complexity index number. This number varies between 1-5, where 1 indicates smooth border while a 5 complexity index shows highly irregular border of the tumor (Figure 1). From each sample, as a mean, 9.5 (ranges 5-14) images were captured and analysed. Similarly a mean of fractal dimensions and number of cells/ clusters was used to estimate a complexity index value.
Figure 1: Human colon biopsies showing tumor growth patterns in colon carcinoma. Figure 1A shows expensive tumor growth with smooth invasive front (low complexity index), Figure 1B indicates the medium complexity index and Figure 1C shows the infiltrative growth pattern with highly coarse invasive front with dispersed tumor cells with high complexity index. (Figure 1D, E and F) are the same images that are thresholded to get tumor area black with white background. (G, H and I) are indicating a tumor invasive front outline during image processing.
Statistical analysis
Statistical analysis was performed by using SPSS version 20 (SPSS Inc., Chicago, IL, USA) p ≤ 0.05 was considered as significant. Kaplan-Meier method was used for survival analysis. To determine a correlation between complexity index and clinicopathological parameters of the patients, Pearson's Chi-squared and Fisher exact test were applied appropriately.
Results
A total of 316 colon carcinoma patients were enrolled in this study. Among these patients, 159 (50.32%) were male and 157 (49.68%) were females. There were 93 (29.43%) patients below 70 years and 223 (70.57%) at age of 70 years or above. According to TNM classification, 4(1.3%) were at T1 stage, 40(12.7%) at T2, 168(53.2%) at T3 and 28(8.9%) were at T4 stage. Similarly, 137(43.4%), 58(18.4%), 44(13.9%) and 1(0.3%) were at N0, N1, N2 and N3 stages respectively. For systemic metastasis (M), 137 (43.4%), 8(2.5%) and 113 (35.76%) were at Mx, M1 and M2 stages. For differentiation of the tumor, 54 (17.09%) were low differentiated, 175(55.38%) were medium differentiated and 72(22.78%) were highly differentiated tumors. When the samples were grouped according to Duke’s classification, 34(10.8%), 119(37.7%), 101(32.0%) and 12(3.8%) cases were at stage A, B, C and D respectively. On dividing samples into right and left colon groups, 179(56.65%) and 59(18.67%) were grouped under right and left colon respectively (Table 1). The information for TNM was missing for 76(24.1%) patients, 15(4.7%) for differentiation, 50(15.8%) for duke’s stages and 78(24.7%) for localization of the tumor.
| Clinicopathological parameters | Number | (% age) | |
|---|---|---|---|
| Age | Age1 | 93 | 29,43 |
| Age2 | 223 | 70,57 | |
| Gender | Male | 159 | 50,32 |
| Female | 157 | 49,68 | |
| Tumor infiltration T | T1 | 4 | 1,27 |
| T2 | 40 | 12,66 | |
| T3 | 168 | 53,16 | |
| T4 | 28 | 8,86 | |
| Lymph node metastasis N | N0 | 137 | 43,35 |
| N1 | 58 | 18,35 | |
| N2 | 44 | 13,92 | |
| N3 | 1 | 0,32 | |
| Systemic metastasis M | MX | 137 | 43,35 |
| M1 | 8 | 2,53 | |
| M2 | 113 | 35,76 | |
| Differentiation | Low | 54 | 17,09 |
| Medium | 175 | 55,38 | |
| High | 72 | 22,78 | |
| Duke’s Stages | A | 34 | 10,76 |
| B | 119 | 37,66 | |
| C | 101 | 31,96 | |
| D | 12 | 3,80 | |
| Localization | Right colon | 179 | 56,65 |
| Left colon | 59 | 18,67 | |
Table 1: Clinicopathological parameters of the samples used diagnosed with colon carcinoma.
Tumor growth pattern and patient survival
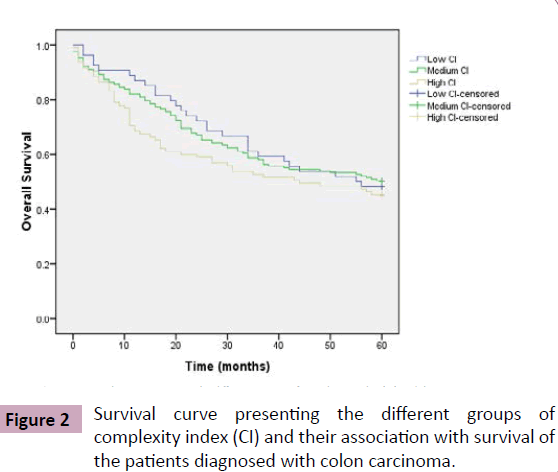
Among 316 patients diagnosed with colon carcinoma, there were 163 (51.58%) deaths. To evaluate prognostic effect of tumor growth pattern on 5 years survival of the patients, the complexity index variable was divided in 3 groups, low complexity index (CI=1), medium complexity index (CI=2 and 3) and high complexity index (CI=4 and 5). There were 54(17.09%) patients with low complexity index, 167 (52.85%) with medium complexity index, and 95 (30.06%) patients were included in the high complexity index group. Comparing survival with these three groups, showed no significantly difference (p=0.522) (Table 2, Figure 2).
| Complexity index | Live (N/%) | Died (N/%) | Total (N/%) | P value |
|---|---|---|---|---|
| Low | 26 (8.23) | 28 (8.86) | 54 (17.09) | 0.522 |
| Medium | 84(26.58) | 83 (26.27) | 167 (52.85) | |
| High | 43(13.61) | 52(16.46) | 95(30.06) | |
| Total | 153 (48.42) | 163 (51.58) | 316 (100) |
Table 2: Association between complexity index and survival of the patients diagnosed with colon carcinoma, Kaplan-Meier’s test.
Tumor growth pattern and clinicopathological data of the patients
To examine whether prognostic relationship of complexity index is influenced by other clinicopathological parameters including age, gender, tumor wall penetration, lymph node metastasis, systemic metastasis, anatomical localization of the tumor, tumor differentiation and dukes stages, we correlated complexity index with these parameters. Significant association of complexity index was confined to gender, systemic metastasis of tumor, localization and differentiation of tumor. Complexity index was significantly higher in females as compared with males (P=0.024). A significant association was found between tumor systemic metastasis and high complexity index (p=0.000). Similarly, tumors at right colon of the patients were significantly associated with complexity index (P=0.002). Another positive correlation was found between tumor differentiation and complexity index (P=0.000). Those patients who were at or above the age of 70 years, were significantly at higher risk of colon carcinoma (p=0.051) Tumor wall penetration, lymph node metastasis and Duke’s stages were not significantly associated with tumor growth pattern; complexity index (with p=0.997, p=0.857, p=783 and p=0.647 respectively) (Table 3).
Discussion
Colorectal cancer is the third most frequent type and 2nd most common cause of cancer related deaths worldwide [23]. The landscape of cancer has dramatically changed in recent years with the understanding and development of novel targeted therapies [24]. In parallel with therapeutic advancement, it is necessary to know about the biological characteristics that determine the prognosis of this disease. Because of heterogeneity of CRC in clinical outcome, great efforts have been made to recognize the histological features that can be used to predict the tumor behaviour and thus selecting the patients for more appropriate therapy.
Many researchers have attempted to find out some prognostic markers in CRC and one of the identified predicting factors is growth pattern of the tumor [7,10]. In this study, we sought to assess whether complexity index of the tumor is associated with survival of the patients diagnosed with colon carcinoma.
We analysed complexity index of 316 patients diagnosed with colon carcinoma and correlated it with survival as well as clinicopathological parameters of the patients. We found that complexity index has a significant association with age (p=0.051), gender (p=0.024), systemic metastasis (p=0.000), differentiation (p=0.000) and localization (p=0.002) of the tumor.
A complexity index is based upon the fractal dimension and number of tumor cells/clusters at the invasive front area of the tumor, where a more irregular tumor growth has a high complexity index value and vice versa. Hahn-Stromberg et al. illustrated that when a tumor has irregular border, its size increases which is compatible with the tumor progression [20]. So an irregular tumor-stromal area has a high complexity index value and ability to metastasize to other organs compared with a regular and smooth invasive border. A similar significant association between complexity index and systemic metastasis has been found in our results that are in consistent with other studies [25].
In addition to metastasis, tumor differentiation was also significantly correlated with complexity index which indicates that as the tumor invasive front becomes more irregular, it is more easily differentiated. Likewise, tumors on the right side of the colon were significantly higher compared to the left side, which is in accordance with previous reports indicating a shift of colon carcinoma from left to right colon with time and age [26,27]. Due to the higher susceptibility of the right colon, complexity index has a significance association with right colon carcinoma.
Our results highlighted a trend of affiliation between the Age 2 group (age ≥ 70 years) and complexity index; this may be because the risk of colon carcinoma increases with age [28].
We also observed a significant association of females being more subjected to higher complexity index than males. Studies show that males are more exposed to colorectal cancer than females and together with high risk, this fact suggests that males are more susceptible to rectal cancer and left sided colon cancer while females are at a higher risk of right-sided colon cancer [29,30]. This discrepancy may be due to the fact that in our study, only colon carcinoma patients were enrolled and rectal carcinoma patients were excluded. Also the number of right-sided tumors was significantly higher in our study.
A previous study indicates that complexity index has a significant association with tumor wall penetration but our findings are not in agreement with those results. It may be due to the large difference in samples size that were analysed in these two studies [21]. Similarly we could not find any association between complexity index and other clinicopathological parameters like lymph node metastasis and tumor duke’s stages. In concordance to our results, Mannan A and Hahn-Strömberg V in 2011 found the same results [21].
We did not find any association between 5 years survival of the patients and complexity index (p=0.522) even though a high complexity index is representative of a more infiltrative type of tumor which in turn is believed to be associated with poor prognosis [31,32]. Recent studies show that adoption of resection and chemotherapy has improved the survival rates, so this anomaly may be due to the fact that some patients with high complexity index/ metastasis got survival benefits when undergoing surgical therapy as was explained by Lodge et al. and Kemeny et al. [5,33].
There are some limitations in this study as; data on colon carcinoma treatment is not available. In addition, the exact cause of mortality as well as the data on cancer resurrection was not available.
Conclusively, our results did not explain the direct relationship between complexity index and survival of the patients diagnosed with colon carcinoma. However complexity index is associated with higher risk of tumor systemic metastasis, which is well known for its prognostic significance. Moreover, we have used 316 colon carcinoma patient samples for tumor growth pattern analysis but the confirmed systemic metastasis information is available for only 121 patients. Thus, further studies with a specific duration of follow up are required to explicate the exact mechanism by which tumor stromal interactions are affecting the behaviour of the tumor.
Author’s Contribution
Abrar Ahmad carried out the image analysis and the statistical analysis. He also performed the sectioning, immunostaining as well as drafted the manuscript. Victoria Hahn-Strömberg conceived idea of the study, design, coordination, funding and helped draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Nyckelfonden, Orebro University Hospital, Orebro, Sweden and Lions Cancer Research Foundation, Uppsala, Sweden for grants funding this study.
References
- Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2: 533-543.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. (2008) Cancer statistics, 2008. CA Cancer J Clin 58: 71-96.
- Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10-29.
- Geoghegan JG, Scheele J (1999) Treatment of colorectal liver metastases. Br J Surg 86: 158-169.
- Lodge JP, Menon KV, Fenwick SW, Prasad KR, Toogood GJ (2005) In-contiguity and non-anatomical extension of right hepatic trisectionectomy for liver metastases. Br J Surg 92: 340-347.
- Yuan Y, Li MD, Hu HG, Dong CX, Chen JQ, et al. (2013) Prognostic and survival analysis of 837 Chinese colorectal cancer patients. World J Gastroenterol 19: 2650-2659.
- Rajaganeshan R, Prasad R, Guillou PJ, Chalmers CR, Scott N, et al. (2007) The influence of invasive growth pattern and microvessel density on prognosis in colorectal cancer and colorectal liver metastases. Br J Cancer 96: 1112-1117.
- Jass JR (1987) The pathological classification of colorectal cancer. Ann Acad Med Singapore 16: 469-473.
- Pinheiro RS, Herman P, Lupinacci RM, Lai Q, Mello ES, et al. (2014) Tumor growth pattern as predictor of colorectal liver metastasis recurrence. Am J Surg 207: 493-498.
- Jass JR, Love SB, Northover JM (1987) A new prognostic classification of rectal cancer. Lancet 1: 1303-1306.
- Hase K, Shatney C, Johnson D, Trollope M, Vierra M (1993) Prognostic value of tumor "budding" in patients with colorectal cancer. Dis Colon Rectum 36: 627-635.
- Wang LM, Kevans D, Mulcahy H, O'Sullivan J, Fennelly D, et al. (2009) Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol 33: 134-141.
- Jass JR, Atkin WS, Cuzick J, Bussey HJ, Morson BC, (1986) The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology, 10:437-459.
- Deans GT, Heatley M, Anderson N, Patterson CC, Rowlands BJ, et al. (1994) Jass' classification revisited. J Am Coll Surg 179: 11-17.
- Cross SS (1994) The application of fractal geometric analysis to microscopic images. Micron 25: 101-113.
- Cross SS (1997) Fractals in pathology. J Pathol 182: 1-8.
- Cross SS, Bury JP, Silcocks PB, Stephenson TJ, Cotton DW (1994) Fractal geometric analysis of colorectal polyps. J Pathol 172: 317-323.
- Landini G, Rippin JW (1993) Fractal dimensions of the epithelial-connective tissue interfaces in premalignant and malignant epithelial lesions of the floor of the mouth. Anal Quant Cytol Histol 15: 144-149.
- Franzén LE, Hahn-Strömberg V, Edvardsson H, Bodin L (2008) Characterization of colon carcinoma growth pattern by computerized morphometry: definition of a complexity index. Int J Mol Med 22: 465-472.
- Hahn-Strömberg V, Edvardsson H, Bodin L, Franzén L (2009) Tumor volume of colon carcinoma is related to the invasive pattern but not to the expression of cell adhesion proteins. APMIS 117: 205-211.
- Mannan A, Hahn-Stromberg V (2012) K-ras mutations are correlated to lymph node metastasis and tumor stage, but not to the growth pattern of colon carcinoma. APMIS 120:459-468.
- Victoria HS, Henrik E, Lennart B, Lennart F (2010) Claudin 1 and Claudin 7 Gene Polymorphisms and Protein Derangement are Unrelated to the Growth Pattern and Tumor Volume of Colon Carcinoma. Int J Biomed Sci 6: 96-102.
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74-108.
- Weiner LM, Dhodapkar MV, Ferrone S (2009) Monoclonal antibodies for cancer immunotherapy. Lancet 373: 1033-1040.
- Van den Eynden GG, Bird NC, Majeed AW, Van Laere S, Dirix LY, et al. (2012) The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin Exp Metastasis 29: 541-549.
- Cucino C, Buchner AM, Sonnenberg A (2002) Continued rightward shift of colorectal cancer. Dis Colon Rectum 45: 1035-1040.
- Saltzstein SL, Behling CA (2007) Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. J Clin Gastroenterol 41: 173-177.
- Patel SA, Zenilman ME (2001) Outcomes in older people undergoing operative intervention for colorectal cancer. J Am Geriatr Soc 49: 1561-1564.
- DeCosse JJ, Ngoi SS, Jacobson JS, Cennerazzo WJ (1993) Gender and colorectal cancer. Eur J Cancer Prev 2: 105-115.
- Blumberg D, Paty PB, Picon AI, Guillem JG, Klimstra DS, et al. (1998) Stage I rectal cancer: identification of high-risk patients. J Am Coll Surg 186: 574-579.
- Krüger S, Noack F, Böhle A, Feller AC (2004) Histologic tumor growth pattern is significantly associated with disease-related survival in muscle-invasive transitional cell carcinoma of the urinary bladder. Oncol Rep 12: 609-613.
- Fukatsu A, Tsuzuki T, Sassa N, Nishikimi T, Kimura T, et al. (2013) Growth pattern, an important pathologic prognostic parameter for clear cell renal cell carcinoma. Am J Clin Pathol 140: 500-505.
- Kemeny NE (2013) Treatment of metastatic colon cancer: "the times they are A-changing". In: J Clin Oncol 31: 1913-1916.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences